
Some experimental evidence, however, suggests that the bonding orbitals on the oxygen are actually unhybridized 2 p orbitals rather than sp 3 hybrids. In this model, the two nonbonding lone pairs on oxygen would be located in sp 3 orbitals. It would seem logical, then, to describe the bonding in water as occurring through the overlap of sp 3-hybrid orbitals on oxygen with 1 sorbitals on the two hydrogen atoms. VSEPR theory also predicts, accurately, that a water molecule is ‘bent’ at an angle of approximately 104.5˚. Recall from your study of VSEPR theory in General Chemistry that the lone pair, with its slightly greater repulsive effect, ‘pushes’ the three N-H sbonds away from the top of the pyramid, meaning that the H-N-H bond angles are slightly less than tetrahedral, at 107.3˚ rather than 109.5˚. The bonding arrangement here is also tetrahedral: the three N-H bonds of ammonia can be pictured as forming the base of a trigonal pyramid, with the fourth orbital, containing the lone pair, forming the top of the pyramid. (It will be much easier to do this if you make a model.)ĭescribe, with a picture and with words, the bonding in chloroform, CHCl 3. In the images below, the exact same methane molecule is rotated and flipped in various positions. Imagine that you could distinguish between the four hydrogens in a methane molecule, and labeled them H a through H d. This system takes a little bit of getting used to, but with practice your eye will learn to immediately ‘see’ the third dimension being depicted.
Normal lines imply bonds that lie in the plane of the page. A dashed wedge represents a bond that is meant to be pictured pointing into, or behind, the plane of the page. In this convention, a solid wedge simply represents a bond that is meant to be pictured emerging from the plane of the page. To do this on a two-dimensional page, though, we need to introduce a new drawing convention: the solid / dashed wedge system. While previously we drew a Lewis structure of methane in two dimensions using lines to denote each covalent bond, we can now draw a more accurate structure in three dimensions, showing the tetrahedral bonding geometry. The length of the carbon-hydrogen bonds in methane is 1.09 Å (1.09 x 10 -10 m). This geometric arrangement makes perfect sense if you consider that it is precisely this angle that allows the four orbitals (and the electrons in them) to be as far apart from each other as possible.This is simply a restatement of the Valence Shell Electron Pair Repulsion (VSEPR) theory that you learned in General Chemistry: electron pairs (in orbitals) will arrange themselves in such a way as to remain as far apart as possible, due to negative-negative electrostatic repulsion.Įach C-H bond in methane, then, can be described as an overlap between a half-filled 1 s orbital in a hydrogen atom and the larger lobe of one of the four half-filled sp 3 hybrid orbitals in the central carbon. The larger lobes of the sp 3 hybrids are directed towards the four corners of a tetrahedron, meaning that the angle between any two orbitals is 109.5 o. Unlike the p orbitals, however, the two lobes are of very different size. The sp 3 hybrid orbitals, like the p orbitals of which they are partially composed, are oblong in shape, and have two lobes of opposite sign. In the new electron configuration, each of the four valence electrons on the carbon occupies a single sp 3 orbital.

In this picture, the four valence orbitals of the carbon (one 2 s and three 2 p orbitals) combine mathematically (remember: orbitals are described by equations) to form four equivalent hybrid orbitals, which are named sp 3 orbitals because they are formed from mixing one s and three p orbitals. In order to explain this observation, valence bond theory relies on a concept called orbital hybridization. How does the carbon form four bonds if it has only two half-filled p orbitals available for bonding? A hint comes from the experimental observation that the four C-H bonds in methane are arranged with tetrahedral geometry about the central carbon, and that each bond has the same length and strength. Recall the valence electron configuration of the central carbon:
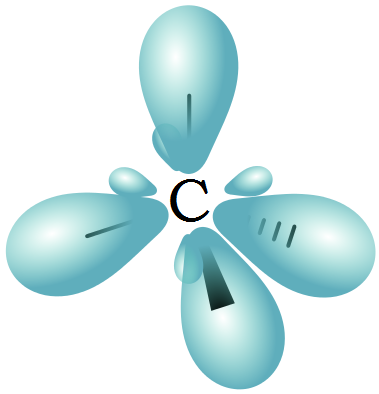
Now let’s turn to methane, the simplest organic molecule. Formation of \(\pi\) bonds - \(sp^2\) and \(sp\) hybridization.Formation of sigma bonds: the H2 molecule.


 0 kommentar(er)
0 kommentar(er)
